
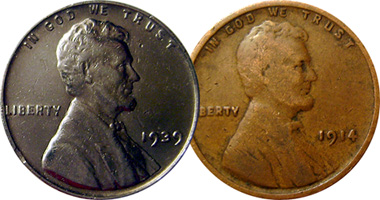
Like all manufacturers, the mint is always looking for better ways to produce their product. Sometimes test coins escape into circulation, and this always causes a stir in the numismatic (coin collector) community. For example, check out this Martha Washingon Test Pattern coin from the US Mint.
Eurofin sent us this picture of an unusual one cent piece he found in circlation. The striking difference in color caught his attention. The 1914 'normal' cent comes from a side-by-side picture that Eurofin took with his camera, so that rules out camera differences.
Not only that, it turns out Eurofin has some sophisticated metallurigcal equipment, and he identified a large amount (about half) of the metal in the coin is nickel, in addition to the normal copper.
After thorough research, we could find no evidence of a nickel test cent produced in 1939. Too bad. If this were the case, Eurofin's piece would be very valuable. More likely, it is worth about one cent, as explained below.
The give-away is the weight. A normal penny weighs 3.11 grams. Eurofin's coin weighs almost exactly that much (within accuracy limits of the scale). If the coin were indeed an alloy of 50 percent nickel and 50 percent copper, its weight would be slightly less than 3.11 grams, since nickel is a little lighter than copper. If it were a plated coin, it would weigh slightly more than 3.11. Extremely accurate scales are needed to do such a weight analysis.
We conclude, therefore, that someone, for some unknown reason, plated Eurofin's coin with nickel. This happens from time to time, and the reason is almost never known.
There is, of course, always a nagging feeling that the research is not adequate. At CoinQuest we are definitely amatuers when it comes to detailed numismatic knowledge. Some people and organizations are *extremely* knowledgable about coins. When this is the case, the best approach is to send your coin to one of these expert organizations: PCGS, NGC, ICG, or ANACS. Do not use others. Look them up on the Internet.
By the way, Eurofin, we contacted Mike Byers Numismatics about your coin. Mike is extremely knowledgable and strongly suggests you send it off to PCGS for authentication and analysis. Hope you do! If you would like, write to CoinQuest or to Mike for information about sending coins to third-party services.
Nickel is only an example of a material that a coin may be plated by. Sometimes, students in high schools do an electrochemical experiment using copper sulfate and sulfuric acid to plate coins in copper. Another common experiment is using silver nitrate and other chemicals to plate coins in silver. There are procedures for plating coins in practically every metal, and many are used as learning experiments in high school classes.
cqLastNotify
About CoinQuest | Privacy Policy | Contact CoinQuest
Copyright 2009 to 2024 CoinQuest.com, all rights reserved.
Daily visitors 91, minutes per visit 6.3, daily coin views 201